Determining Ka by the half titration of a weak acid is a crucial technique in analytical chemistry. This method provides an accurate and efficient way to determine the Ka value, which is a measure of the strength of a weak acid.
Understanding Ka values is essential for various chemical processes and has applications in chemistry, biology, and other fields.
This comprehensive guide delves into the principles, experimental setup, data analysis, and factors affecting Ka determination using half titration. By exploring these concepts, we gain insights into the significance of Ka values and their applications in understanding chemical reactions.
Understanding the Concept of Determining Ka by Half Titration
Ka is the acid dissociation constant, a measure of the strength of an acid. Determining Ka for a weak acid can be achieved using half titration. This method relies on the principle that at half-equivalence point, the concentration of the weak acid is equal to its conjugate base.
By measuring the pH at this point, we can determine the pKa and subsequently calculate the Ka value.
Experimental Setup and Titration Procedure
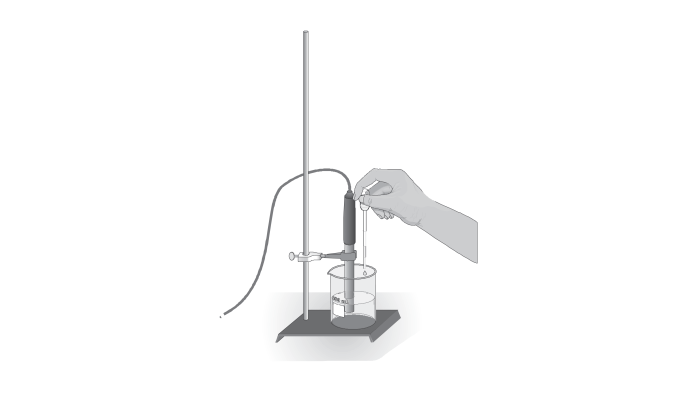
Experimental Setup
The experimental setup for half titration requires a burette, a conical flask, a pH meter, and solutions of the weak acid, strong base, and distilled water.
Titration Procedure
- Prepare a known concentration of the weak acid solution.
- Fill the burette with the strong base solution.
- Add a known volume of the weak acid solution to the conical flask.
- Titrate the weak acid solution with the strong base solution while monitoring the pH using the pH meter.
- Record the volume of the strong base solution added at the half-equivalence point, where the pH is equal to the pKa of the weak acid.
Data Analysis and Calculations
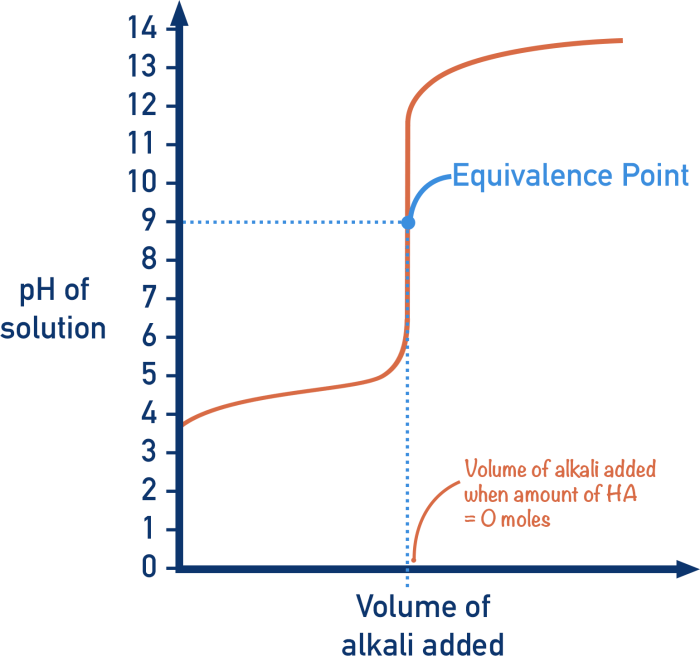
Determining Equivalence Point
The equivalence point is determined by plotting the pH versus the volume of strong base added. The equivalence point is the point at which the pH is equal to the pKa of the weak acid.
Calculating Ka
Once the equivalence point is determined, the Ka can be calculated using the following formula:
Ka = [H+][A-] / [HA]
where [H+] is the hydrogen ion concentration, [A-] is the conjugate base concentration, and [HA] is the weak acid concentration.
Factors Affecting Ka Determination
Temperature, Determining ka by the half titration of a weak acid
Temperature affects the Ka of a weak acid. As temperature increases, the Ka value also increases.
Ionic Strength
Ionic strength can affect the Ka of a weak acid. High ionic strength can decrease the Ka value.
Presence of Other Acids or Bases
The presence of other acids or bases can affect the Ka of a weak acid. Strong acids can suppress the dissociation of weak acids, while strong bases can promote it.
Applications of Ka Determination
Chemistry
- Predicting the pH of solutions
- Determining the solubility of sparingly soluble salts
- Understanding acid-base equilibria
Biology
- Determining the pH of biological fluids
- Understanding enzyme activity
- Studying protein folding
Essential FAQs: Determining Ka By The Half Titration Of A Weak Acid
What is the principle behind determining Ka using half titration?
Half titration involves titrating a weak acid with a strong base to its half-equivalence point, where half of the weak acid has been neutralized. At this point, the concentration of the conjugate base is equal to the concentration of the remaining weak acid, allowing for the direct calculation of Ka using the Henderson-Hasselbalch equation.
How does pH affect Ka determination?
pH plays a crucial role in Ka determination. The equivalence point, where the concentration of the conjugate base equals the concentration of the weak acid, occurs at a pH equal to the pKa of the weak acid. Therefore, measuring the pH at the equivalence point allows for the determination of pKa and, subsequently, Ka.